Mammalian Toxicology
Mammalian Toxicology
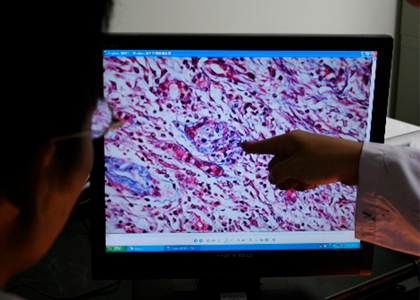
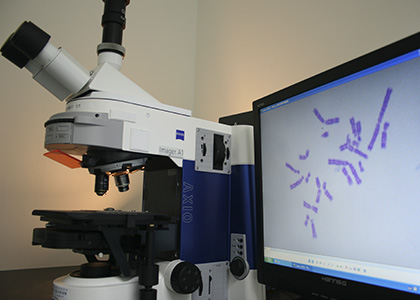
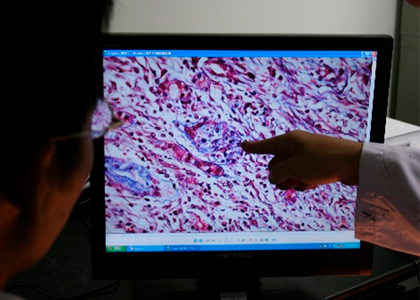
Level CRO service has good experience in supporting clients with excellence in the management of toxicology studies in rodent and non-rodent species, all accompanied by on-time reporting. A certified team of study directors work closely with our clients to ensure that studies are conducted to meet both product development and international regulatory needs. The service is supported by a dedicated pathology group and laboratory animal specialists. In addition, all protocols are designed to meet the regulatory requirements of the various countries in which the products are to enter clinical trials or be marketed.
- Service
Service
Single dose toxicity
- Acute toxicity study
- Dose range finding study
Repeated dose toxicity
- 14 day or 28 day subacute toxicity study
- 90 day subchronic toxicity study
- 180 day~1 year chronic toxicity study
Carcinogenicity
- 2 year toxicity /carcinogenicity rodents
- carcinogenicity in transgenic animal models
Immunotoxicity
Reproductive toxicity
- Multi-generation study
- Teratology (embryo toxicity) study in rat or rabbit
Toxicokintics